Friedländer annulation: scope and limitations of metal salt Lewis acid catalysts in selectivity control for the synthesis of functionalised quinolines†
Abstract
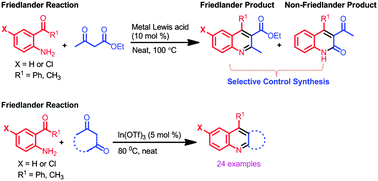
The scope and limitations of metal salt Lewis acid catalysts were examined for the selectivity control for the formation of Friedländer and non-Friedländer products during the reaction involving 2-aminobenzophenone and ethyl acetoacetate. Among a pool of metal halides, tetrafluoroborates, perchlorates, and triflates used as catalysts, In(OTf)3 emerged as the most effective catalyst for the selective/exclusive formation of the Friedländer product. The generality of the In(OTf)3-catalysed Friedländer reaction was demonstrated by the reaction of differently substituted 2-aminoarylketones with various carbonyl compounds containing an active methylene group (e.g., β-ketoesters, cyclic/acyclic β-diketones, cyclic/acylic ketones, and aryl/heteroaryl methyl ketones) under solvent-free conditions affording the desired quinolines in 75–92% yields.

 Please wait while we load your content...
Please wait while we load your content...