Synthesis of a fluorinated Ezetimibe analogue using radical allylation of α-bromo-α-fluoro-β-lactam†
Abstract
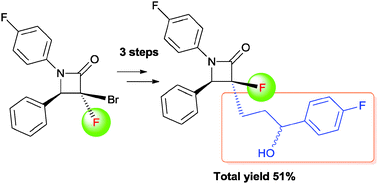
The synthesis of an α-fluoro-β-lactam-containing Ezetimibe analogue was accomplished starting from α-bromo-α-fluoro-β-lactam which was readily prepared from ethyl dibromofluoroacetate. A facile and efficient method for the introduction of the C3 alkyl side chain was realized via radical allylation. The diastereoselective allylation of α-bromo-α-fluoro-β-lactam was successfully applied to construct the relative configuration of the β-lactam nucleus between C3 and C4. Further modification of the allyl side chain gave the 3′-(4-fluorophenyl)-3′-hydroxypropyl group through Wacker oxidation and nucleophilic arylation.

 Please wait while we load your content...
Please wait while we load your content...