Synthesis, structures and magnetism of a series of dinuclear and one-dimensional Ni–Ln complexes: single-molecule magnetic behavior in one-dimensional nitrate-bridged Dy analogue†
Abstract
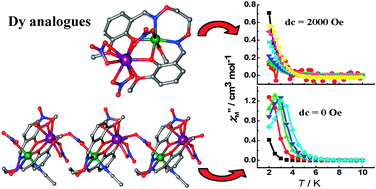
By diffusion of methyl tert-butyl ether vapor into an acetonitrile solution containing [Ni(L)(H2O)2] (L = 1,2-bis(3-methoxy-salicylideneaminooxy)ethane) and Ln(NO3)3·6H2O, three Ni–Ln heterometallic complexes, [Ni(μ-L)(MeCN)2Ln(NO3)3] (Ln = Gd for 1, Tb for 2), and [Ni(μ-L)(MeCN)Dy(NO3)2(μ-NO3)·MeCN]n (3) were obtained. When the reaction was carried out in methanol/chloroform (2 : 1) containing H2L, Ni(Ac)2·4H2O and Ln(NO3)3·6H2O with diffusion of diethyl ether vapor, two Ni–Ln heterometallic complexes [Ni(μ-L)(CH3OH)(μ-NO3)Ln(NO3)2] (Ln = Tb for 4, and Dy for 5) were synthesized. The X-ray analyses demonstrated that complexes 1, 2, 4 and 5 display 3d–4f heterodinuclear structures, and that the NiII and LnIII ions are bridged by diphenolato atoms for 1 and 2, and by a syn–syn nitrate group and diphenolato atoms for 4 and 5. Compound 3 is made of a rare anti–anti nitrate- and phenolate-bridged Ni–Dy heterometallic chain. All complexes exhibit a net ferromagnetic interaction between the NiII and LnIII ions. Alternating current susceptibility measurements demonstrated that compound 3 behaved as a single-molecule magnet with an effective energy barrier of 15.8 K under a zero direct current field, however, 5 only displayed slow magnetic relaxation under a 2000 Oe direct current field.

 Please wait while we load your content...
Please wait while we load your content...