Synthesis and acid–base properties of a proton-bridged biaryl compound based on pyridylazulene†
Abstract
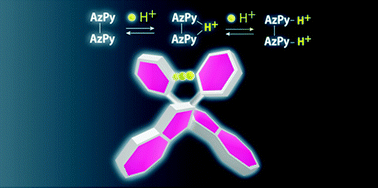
Herein, we report the synthesis of 1,1′-bi(2-pyridylazulene) (1), in which pyridyl moieties were coupled to a biaryl framework for hydrogen bonding between the two aryl skeletons. The two 2-pyridylazulene moieties in 1 were linked through facile aryl–aryl coupling between the 1- and 1′-positions of the azulene skeletons, where 1-haloazulene was stabilized by electron withdrawing pyridyl substitution. In addition, single crystals of the mono-protonated species (1H+) were successfully obtained as the BF4− salt. X-ray diffraction analysis at 153 K revealed an intramolecular hydrogen bond between the two pyridyl moieties, giving a racemic mixture of axial chiral species. DFT calculations were performed to understand the hydrogen bonding structure and an almost single minimum potential for thermal proton motion was suggested. The acid–base characteristics were investigated in acetonitrile and 1 was revealed to exhibit two-step protonation of its pyridyl moieties. By comparison with monomeric 2-pyridylazulene, the stronger basic character of 1 was confirmed. This is ascribed to the macrocyclic effect of the two pyridyl moieties bridged by a proton, as seen in the single crystal of 1H+. Furthermore, two different energy shifts associated with intramolecular transition were observed under protonation; the first protonation produced a blue shift in the absorption maximum, whereas a red shift was observed for the second protonation. The unusual blue shift was explained by the stabilization of the HOMO owing to an extended electronic structure between the two azulene skeletons. This unique steric structure was achieved by proton bridging in the pyridyl-substituted biaryl compound.

 Please wait while we load your content...
Please wait while we load your content...