Palladium catalyzed decarboxylative acylation of arylboronic acid with ethyl cyanoacetate as a new acylating agent: synthesis of alkyl aryl ketones†
Abstract
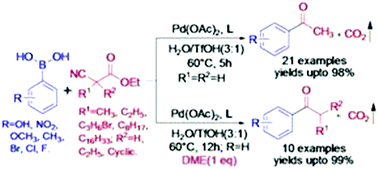
Palladium catalyzed acylation of arylboronic acid containing various functional groups was performed efficiently by ethyl cyanoacetate/substituted ethyl cyanoacetate as the acylating agent in aqueous triflic acid medium. The alkyl aryl ketones were obtained in good to excellent yields, first by addition of arylboronic acid to the nitrile group of ethyl cyanoacetate and their derivatives, followed by in situ decarboxylation of the resulting β-ketoester.

 Please wait while we load your content...
Please wait while we load your content...