The reactivity of manganese dioxide towards different substrates in organic solvents†
Abstract
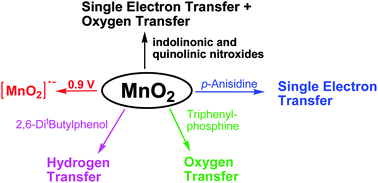
The reactivity of manganese dioxide in organic solvents was studied by reacting commercial manganese dioxide MnO2 with different substrates. The reaction of MnO2 with arylimino, indolinonic and quinolinic aromatic nitroxides gave quinoneimine N-oxides via an electron transfer process followed by oxygen transfer. A reduction potential of 0.9 V vs. NHE was roughly estimated for manganese dioxide in aprotic organic solvents by considering the oxidation potentials of those nitroxides able to react with MnO2. By the reaction with para-anisidine the amine radical cation was initially formed through a simple electron transfer process. In the case of substituted phenols, the isolated products were derived instead from the phenoxy radicals generated via hydrogen transfer or an equivalent process. Finally, the quantitative conversion of triphenylphosphine to triphenylphosphine oxide was explained by oxygen atom transfer in a cyclic intermediate derived from an initial nucleophilic attack of phosphorus on MnO2.

 Please wait while we load your content...
Please wait while we load your content...