Oxidation of 3,5-di-tert-butylcatechol and 2-aminophenol by molecular oxygen catalyzed by an organocatalyst†
Abstract
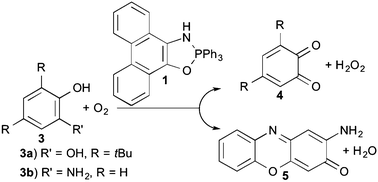
1,3,2-Oxazaphospholes are able to catalyze the oxidation of 3,5-di-tert-butylcatechol with 3O2 to the corresponding o-quinone and 2-aminophenol to 2-aminophenoxazine-3-one in methanol. In both the cases, an overall third order reaction rate equation and a new type of biomimetic organocatalyst for oxidation reactions was found. A one electron transfer of the phenolate, which is formed through the deprotonation of the substrates by the catalyst, to dioxygen seems to be rate-determining step.

 Please wait while we load your content...
Please wait while we load your content...