A green approach for the synthesis of bis (substituted sulfabenzamide) para-benzoquinone based on the reaction of sulfabenzamide with electrochemically generated para-benzoquinone and its antibacterial evaluation†
Abstract
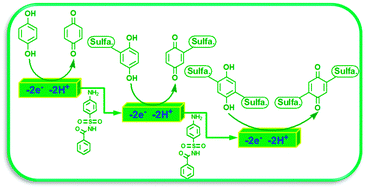
The electrochemical synthesis of a new bis (substituted sulfabenzamide) para-benzoquinone (5) was carried out via the electrochemical oxidation of hydroquinone (1) in the presence of sulfabenzamide (4-amino-N-benzoyl-benzenesulfonamide) (2). A plausible mechanism for the oxidation of 1 in the presence of 2 is presented. Hydroquinone (1) was converted into 5via an ECECE mechanism. The electrochemical synthesis of 5 was successfully performed in one-pot, in a water/ethanol mixture under green conditions without the use of toxic reagents and solvents. Compound 5 was evaluated for its in vitro antibacterial activity against Escherichia coli (E. coli) ATCC 35218 (Gram-negative) and Staphylococcus aureus (S. aureus) ATCC 6538 (Gram-positive). It was found that the tested compound was more active against Gram-positive than Gram-negative bacteria.

 Please wait while we load your content...
Please wait while we load your content...