Quantitative and highly selective sensing of sodium houttuyfonate via long-aliphatic chains hydrophobic assembly and aggregation-induced emission†
Abstract
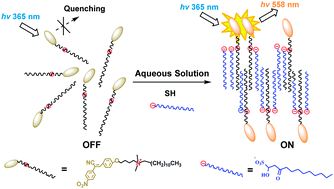
A new α-cyanostilbene derivative, CDB-DMA12, was designed and synthesized as a supramolecular chemosensor for the detection of sodium houttuyfonate (SH) via aggregation-induced emission enhancement (AIEE), which could efficiently bind with SH, then induce an obvious fluorescence enhancement and visible absorption red-shifting (by the naked eye). Accordingly, a linear relationship was found when plotting the fluorescence intensity at 558 nm against SH concentration, with an estimated detection limit of 8.5 μM. Interestingly, morphology changes from nanoparticles to sheet, scroll and tube-shaped aggregates were observed on CDB-DMA12 after the addition of SH. Moreover, CDB-DMA12 showed high selectivity to SH among other sulfonyl containing species, which is attributed to the synergistic effect of its quaternary ammonium, the hydrogen bonding of the active group sites and the twelve carbon containing long-aliphatic chain units. In addition, the results paved a new way for the detection/recognition of amphiphilic natural products in aqueous solution with high sensitivity and selectivity. Besides, it provided a possible approach for the preparation of new fluorescent micro/nano-scaled architectures through the solution self-assembly of oppositely-charged amphiphiles.

 Please wait while we load your content...
Please wait while we load your content...