The effect of a hydrogen bond on the supramolecular self-aggregation mode and the extent of metal-free benzoxazole-substituted phthalocyanines†
Abstract
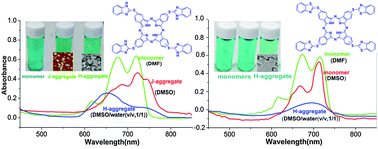
Two novel metal-free phthalocyanines have been designed and synthesized, namely tetra{[1H-benzo(d)imidazol-2-yl]thiol}phthalocyanine (TBIT-Pc) and tetra{[benzo(d)thiazol-2-yl]thiol}phthalocyanine (TBTT-Pc). These two compounds showed similar structures, while imidazolyl-NH in the substitutes of TBIT-Pc could form more hydrogen bonds. TBIT-Pc and TBTT-Pc were fully characterized by elemental analysis, 1H NMR, MALDI-TOF MS, FT-IR and the UV-Vis absorption spectrum. The self-assembly properties of TBIT-Pc and TBTT-Pc were comparatively studied. TBIT-Pc and TBTT-Pc were present as monomers in DMF in the concentration range of 9.04–20.3 μM. Depending mainly on the intermolecular hydrogen bonding (N–H⋯N) between benzimidazole substitutes, “head-to-tail” J-aggregates of TBIT-Pc were formed in DMSO, while there was no aggregation of TBTT-Pc in the same solvent. “Face-to-face” H-aggregates of TBIT-Pc and TBTT-Pc were formed with the addition of water to the solutions of DMSO, and the degree of aggregation increased with the introduction of H-bonds (N–H⋯N) in the benzimidazole substitutes of TBIT-Pc. The atomic force microscope (AFM) image and dynamic light scattering (DLS) displayed the formation of well-defined nanoparticles with a diameter of ca. 30 ± 15 nm with J-type aggregation of TBIT-Pc. And the dendritic nanostructures with H-aggregates of TBIT-Pc and TBTT-Pc with different size were observed from transmission electron microscopy (TEM) images. The possible mechanism of the effect of H-bonds on the formation of J-aggregates of TBIT-Pc and the H-aggregation of TBIT-Pc and TBTT-Pc was also discussed. In the formation process of aggregates, H-bonds and π–π interaction may be the dominant factors. In addition, the nanostructures fabricated from TBIT-Pc and TBTT-Pc showed good semiconducting properties revealed by current–voltage measurements.

 Please wait while we load your content...
Please wait while we load your content...