Regiochemistry of nucleophilic substitution of pentachloropyridine with N and O bidentate nucleophiles†
Abstract
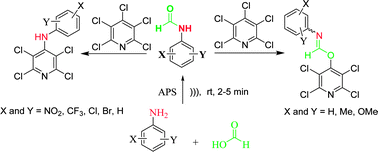
Site reactivity of some enol–imines derived from N-aryl formamides with pentachloropyridine under basic conditions in dry CH3CN was investigated. The aromatic nucleophilic substitution of pentachloropyridine with enol–imines occurs at the 4-position of pyridine ring by both oxygen and nitrogen site of enol–imines. Nucleophilic attack by the oxygen of enol–imine gave corresponding oximino compounds as a mixture of E- and Z-isomers. In contrast, nucleophilic attack by the nitrogen of enol–imine gave the unexpected N,N-di-substituted aryl compounds. The structures of all the compounds were confirmed by IR, 1H NMR, 13C NMR and 19F NMR spectroscopy as well as elemental analysis and X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...