Generating active and non-selective oxidizing species by previous treatment of niobium-doped mesoporous silica with hydrogen peroxide
Abstract
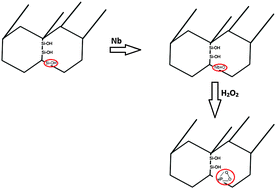
Active catalysts for oxidation of non-selective organic compounds were produced by doping silica with niobium followed by treatment of Nb-doped mesoporous silica with hydrogen peroxide. Low angle XRD patterns revealed that niobium replaced silicon in the silica framework. TEM images confirmed the isomorphic substitution as indicated by the increase of the interplanar spacing after incorporation with Nb in the silica framework. The H2O2 treatment strongly affected the morphology of Nb-doped silica, but EDS and XPS measurements showed that the niobium is maintained in the silica structure even after H2O2 treatment. The isomorphic substitution of Si4+ by Nb5+ in the silica framework can generate acidic sites on the Nb-doped silica, which can contribute to the electron transfer in the oxidation process of organic compounds. Catalytic tests using rhodamine B as a model molecule of textile effluents showed that both processes of Nb doping and treatment with H2O2 are essential to obtain a highly active and oxidizing system. The better performance of Nb-doped silica treated with H2O2 was assigned to formation of niobium-peroxo groups, which act as excellent oxidizing sites.

 Please wait while we load your content...
Please wait while we load your content...