Extraction and mechanistic investigation of trace dibutyl phthalate using an ionic liquid aqueous two-phase system
Abstract
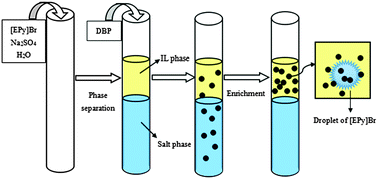
A novel, economical and sensitive sample pretreatment technique, together with the ionic liquid aqueous two-phase system, was established to extract and determine dibutyl phthalate at trace levels. This technique employed high-performance liquid chromatography coupled with ultraviolet detection. Samples were extracted using a mixture of ionic liquid N-ethyl pyridinium bromide and sodium sulfate salt. The factors affecting extraction, including the amount of components, temperature, pH and extraction time, were optimized. Under the optimized conditions, over 97% of the dibutyl phthalate in standard solution samples could be extracted. The limit of detection and the limit of quantification for the analyses are 0.25 ng mL−1 and 1.0 ng mL−1, respectively, with relative standard deviations ranging from 3.7% to 6.9%. This method was practical when applied to the analysis of dibutyl phthalate in beer, wine and spirit samples, and the recovery of dibutyl phthalate was 91.2–100.4%. The ionic liquid aqueous two-phase system was shown to be much simpler and more environmentally friendly for the determination of trace dibutyl phthalate and for other small biomolecules in water samples.

 Please wait while we load your content...
Please wait while we load your content...