Pd-catalysed ortho-alkoxylation of benzamides N-protected with an iminophosphorane functionality
Abstract
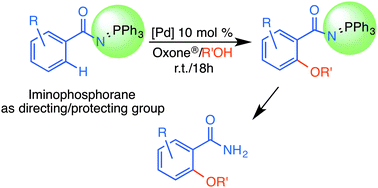
The oxidative coupling of keto-stabilised iminophosphoranes (IPs) Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC(O)C6HxRy with alcohols R′OH affords the alkoxylated species Ph3P
NC(O)C6HxRy with alcohols R′OH affords the alkoxylated species Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC(O)C6Hx−1Ry-2-OR′. The process is catalysed by 10% PdCl2(NCMe)2, uses oxone® as an oxidant and alcohol R′OH as a source of OR′ groups and reaction solvent. The reaction takes place at room temperature regioselectively at the ortho-position of the benzamide ring, gives only the mono-alkoxylated derivatives, and shows tolerance to a variety of functional groups and different primary and secondary alcohols. Better yields were obtained when the aryl ring contains electron-releasing substituents (OMe, Me). The iminophosphorane moiety plays a dual role as the protecting/directing group, and can be hydrolysed to give the corresponding free benzamide.
NC(O)C6Hx−1Ry-2-OR′. The process is catalysed by 10% PdCl2(NCMe)2, uses oxone® as an oxidant and alcohol R′OH as a source of OR′ groups and reaction solvent. The reaction takes place at room temperature regioselectively at the ortho-position of the benzamide ring, gives only the mono-alkoxylated derivatives, and shows tolerance to a variety of functional groups and different primary and secondary alcohols. Better yields were obtained when the aryl ring contains electron-releasing substituents (OMe, Me). The iminophosphorane moiety plays a dual role as the protecting/directing group, and can be hydrolysed to give the corresponding free benzamide.

 Please wait while we load your content...
Please wait while we load your content...