Electrochemical generation of a Michael acceptor: a green method for the synthesis of 4-amino-3-(phenylsulfonyl)diphenylamine derivatives†
Abstract
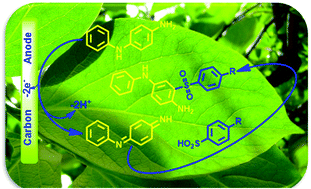
Electrochemical oxidation of 4-aminodiphenylamine in aqueous solution and in the presence of some arylsulfinic acids as nucleophiles was studied and its reaction mechanism was discussed. Using the voltammetric data, a one-pot and environmentally friendly electrochemical approach for the synthesis of 4-amino-3-(phenyl sulfonyl)diphenylamine derivatives via the Michael type addition reaction of anodically generated N-phenylquinonediimine with arylsulfinic acids, at a carbon electrode, without toxic reagents and solvents is reported.

 Please wait while we load your content...
Please wait while we load your content...