Microwave promoted C–O coupling for synthesizing O-aryloxytriazole nucleoside analogues†
Abstract
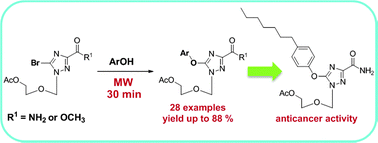
Synthetic nucleoside analogues with novel structural entities are of considerable importance in the search for new structural paradigms with biologically interesting activities. We report in this work that microwave promoted C–O coupling reaction is able to efficiently offer a large array of novel O-arylated triazole nucleosides with considerably improved yields and appreciably reduced reaction time. One of the synthesized compounds showed interesting anticancer activity against human prostate and pancreatic cancers. The synthetic method described in this work not only highlights the importance of microwave irradiation in organic synthesis but also provides a promising access towards synthesizing novel nucleoside analogues which can offer molecular diversity in the quest for new and potential drug candidates.

 Please wait while we load your content...
Please wait while we load your content...