A selective ratiometric fluoride ion sensor with a (2,4-dinitrophenyl)hydrazine derivative of bis(indolyl) methane and its mode of interaction†
Abstract
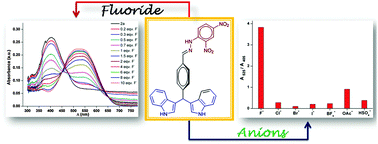
In this report, a new easy-to-synthesize chemosensor, a (2,4-dinitrophenyl)hydrazine (DNP) derivative of 4-(di(1H-indol-3-yl)methyl)benzaldehyde (hereafter 2a), was designed, synthesized and employed as a selective optical chemosensor for fluoride through naked eye detection via patterns of color changes as well as changes in absorption signals. The binding interaction between 2a and fluoride from 1H NMR, UV-vis, and density functional studies suggests that fluoride-induced interaction followed by deprotonation to its corresponding tri-anions is responsible for the significant color and spectral changes in the absorption properties of 2a. The ratiometric responses of 2a specifically to fluoride ions allow us to detect and estimate the concentration of fluoride ions accurately up to 2 μM.

 Please wait while we load your content...
Please wait while we load your content...