Introducing “carborazine” as a novel heterocyclic aromatic species†
Abstract
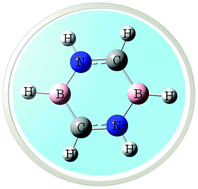
Borazine is regarded as inorganic benzene, possessing a number of features analogous to aromatic benzene. The aromaticity of borazine, however, is a controversial matter to date. We propose a new heterocyclic species by introducing C, B and N atoms at opposite faces of a six-membered ring. This novel species, which we refer to as “carborazine,” possesses C2h symmetry, and more importantly it is aromatic. The aromaticity of carborazine is established by NICS values and supported by a number of topological parameters. Unlike borazine and benzene, carborazine possesses non-degenerate molecular orbitals and a relatively smaller HOMO–LUMO gap. Finally, we show the formation of phenol, aniline (by substitution) and naphthalene analogues (by fusion) of carborazine, which may suggest that a new class of heterocyclic species can be obtained using carborazine as building block.


 Please wait while we load your content...
Please wait while we load your content...