Synthesis of novel sulfonamide azoles via C–N cleavage of sulfonamides by azole ring and relational antimicrobial study†
Abstract
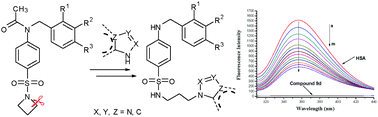
A series of novel sulfonamide azoles were synthesized via C–N cleavage of sulfonamides by an azole ring. This type of reaction could be performed smoothly in DMF and some influential factors including temperature, solvent and reaction time to this reaction were also investigated. The antimicrobial results revealed that sulfonamide 9d bearing a 2-methyl-5-nitroimidazole moiety gave the best anti-P. aeruginosa efficiency in this series and was equivalent to chloromycin (MIC = 16 μg mL−1). The combination of sulfonamide azole 9d with chloromycin, norfloxacin, or fluconazole gave significant synergistic effects. Further research showed that compound 9d could effectively intercalate into calf thymus DNA to form the 9d–DNA complex, which might block DNA replication to exert its powerful antimicrobial activity. The transportation behavior of this compound using human serum albumin (HSA) demonstrated that the electrostatic interactions played major roles in the strong association of compound 9d with HSA.

 Please wait while we load your content...
Please wait while we load your content...