Comment on “The role of electrostatic induction in secondary isotope effects on acidity” by E. A. Halevi, New J. Chem., 2014, 38, 3840
Abstract
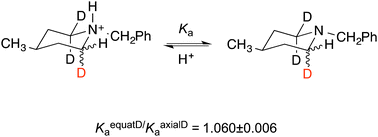
We reaffirm our conclusion that secondary deuterium isotope effects on acidity are due to n–σ* delocalization that decreases vibrational frequencies and zero-point energies. We reject an electrostatic or inductive explanation that arises from the anharmonicity of the C–H bond. We address calculated values of atomic charges, dipole moments, and dipole-moment derivatives dμ/dr, and we show the isotope effect to be a stereoelectronic phenomenon arising from harmonic vibrations.

 Please wait while we load your content...
Please wait while we load your content...