One-pot synthesis of secondary amines from alcohols and nitroarenes on TiO2 loaded with Pd nanoparticles under UV irradiation†
Abstract
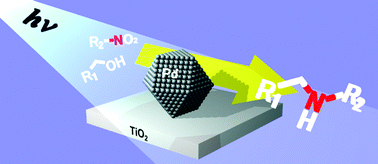
Photoirradiation (λ > 300 nm) of TiO2 loaded with Pd nanoparticles (2 wt%, ca. 5 nm diameter) in water containing benzyl alcohol and nitrobenzene at room temperature successfully produces the corresponding secondary amine (N-benzylaniline) with 96% yield. This is achieved via three consecutive catalytic reactions: (i) photocatalytic oxidation of alcohol (aldehyde formation) and reduction of nitrobenzene (aniline formation); (ii) catalytic condensation of the formed aldehyde with aniline on the TiO2 surface (imine formation); and, (iii) photocatalytic hydrogenation of the formed imine (secondary amine formation). This catalytic system successfully produces several kinds of secondary amines, even those containing reducible substituents such as –CN, –COOH, or –CHO with >76% yields.

 Please wait while we load your content...
Please wait while we load your content...