Investigation of the copper(i) catalysed azide–alkyne cycloaddition reactions (CuAAC) in molten PEG2000†
Abstract
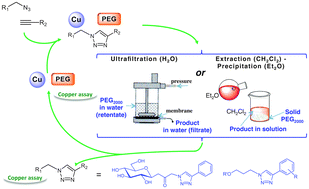
The copper-catalysed azide–alkyne cycloaddition of lipophilic and hydrophilic substrates was investigated in molten PEG2000 using various sources of Cu(I). The reaction proceeded smoothly and afforded the corresponding triazole in good yields. Depending on the solubility of the product, two types of treatment were investigated: precipitation of the polymer-solvent for the compounds soluble in organic solvents, and ultrafiltration for the water-soluble compounds. In the former case, assays demonstrated that most of the copper precipitated together with PEG, thus enabling the efficient recycling of PEG–Cu(I), while for hydrophilic compounds, the treatment led to the precipitation of copper. The electron paramagnetic resonance spectroscopy study showed that in this chelating solvent copper was protected from oxidation to Cu(II), therefore, in PEG the reaction could be carried out under an uncontrolled atmosphere. Upon examination of copper turnings surface by X-ray photoelectron spectroscopy, it was also demonstrated that the nature of the substrates had an influence on the species of copper dissolved in the PEG, which led to different results in terms of reactivity when copper turnings were used as source of Cu(I).

 Please wait while we load your content...
Please wait while we load your content...