A highly efficient and reusable MCM-41-immobilized bipyridine copper(i) catalyst for the C–Se coupling of organoboronic acids with diaryl diselenides†
Abstract
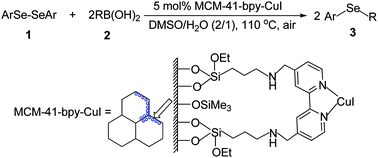
A highly efficient MCM-41-immobilized bipyridine copper(I) complex [MCM-41-bpy-CuI] was prepared from 4,4′-bis[3-(triethoxysilyl)propylaminomethyl]-2,2′-bipyridine via immobilization on the mesoporous material MCM-41, followed by reaction with CuI. In the presence of 5 mol% MCM-41-bpy-CuI, the cross-coupling reaction of organoboronic acids with diaryl diselenides proceeded smoothly in DMSO/H2O (2/1) at 110 °C under air to afford a variety of diorganyl selenides in good to excellent yields. This heterogeneous copper catalyst can be recovered and recycled by a simple filtration of the reaction solution and used for at least 10 consecutive trials without any decrease in activity.

 Please wait while we load your content...
Please wait while we load your content...