Synthesis, characterization, and physical properties of a poly(acrylamide-co-4-cyanobiphenyl-4′-oxyundecylacrylate)
Abstract
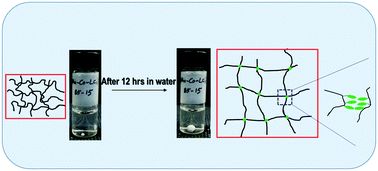
A series of copolymers containing 4-cyanobiphenyl-4′-oxyundecylacrylate (LC11) as the hydrophobic liquid crystal (LC) unit and acrylamide (AAm) as the hydrophilic unit were synthesized by free radical polymerization. 1H NMR spectra of the copolymers confirmed the successful copolymerization. The PAAm-co-PLC11 (75/25), (80/20), and (85/15) copolymers showed swelling behavior in water and formed hydrogels because of the hydrophilic AAm units in the copolymer chains while the LC11 units formed cross-linking junctions by organizing into microstructures. The organization of the LC11 units into microstructures was revealed by a polarized optical microscopy (POM) study of the copolymer hydrogels. The copolymers with LC11 ratios higher than 25 mol% were too hydrophobic to swell in water to form hydrogels. This might be because of the molecular weight of the LC11 units, which is several fold higher than AAm. The copolymers with less than 15 mol% LC11 were so hydrophilic that water dissolved them. The POM and wide angle X-ray scattering (WAXS) studies of the copolymer fiber revealed that the LC11 units were aligned with the mesogens' direction, which is perpendicular to the fiber axis.

 Please wait while we load your content...
Please wait while we load your content...