Predicting self-assembly and structure in diluted aqueous solutions of modified mono- and bis-β-cyclodextrins that contain naphthoxy chromophore groups†
Abstract
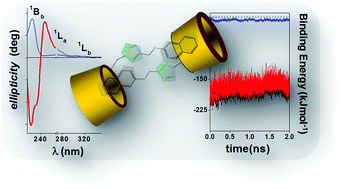
The water diluted solution behaviour of mono- and bis-β-cyclodextrin (mono- and bis-CD) derivatives, whose appended groups and inter-CD linkers contain a naphthoxy chromophore moiety, has been studied using steady-state and time-resolved fluorescence techniques, circular dichroism and molecular modelling. Mono-CD derivatives form non-covalent dimeric tail-to-tail supramolecular structures via the mutual partial penetration, through their primary sides, of axially oriented naphthoxy appended groups and the self-inclusion of the naphthoxy moiety is rather improbable. Non-covalent dimer formation may compete with any guest complexation. Nevertheless, these assemblies can be broken up by decreasing medium polarity or when the appended group is captured by macrorings such as cucurbit[7]urils or native βCDs. Bis-CD derivatives, however, do not exhibit self-association processes which were observed in the mono-derivatives. This is because the presence of the bulky naphthoxy group in the spacer keeps the βCD cavities, which are capable of accommodating an external guest, away from each other. The dinaphthoxy group, in the bis-NβCD, was located in a quasi-parallel plane conformation between both CDs.

 Please wait while we load your content...
Please wait while we load your content...