Spin crossover and high spin electroneutral mononuclear iron(iii) Schiff base complexes involving terminal pseudohalido ligands†
Abstract
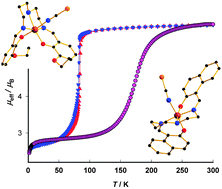
Investigations into a series of six novel mononuclear iron(III) Schiff base complexes with the general formula [Fe(L)X] (where H2L is a pentadentate Schiff-base ligand, X = pseudohalido ligand) are reported. Several different aromatic 2-hydroxyaldehyde derivatives were used in combination with N,N′-bis(2-aminoethyl)-1,3-propanediamine (compounds 1–5) and 2,2′-diaminodiethylamine (for 6) to synthesize the H2L Schiff base ligands. The consecutive reactions with iron(III) chloride resulted in the preparation of the [Fe(L)Cl] precursor complexes which were left to react with pseudohalido ligands (NCS− for 1, 2, 3, 4, 6; N3− for 4). Structural investigations revealed a usual coordination of the pentadentate Schiff base ligands via N3O2 donor atoms and the sixth coordination place is occupied by the N donor of the corresponding pseudohalido ligand. The spin crossover was observed in two cases with transition temperatures: Tc = 83 K (hysteresis width of ΔT = 2 K) for 1 and Tc = 174 K for 2. Magnetic investigations of compounds 3–6 revealed high spin behaviour. The magnetic data of all compounds were analysed using the spin Hamiltonian formalism including the zero-field splitting term and the molecular field effect. In the case of the spin crossover complexes 1 and 2, the Ising-like model was applied.


 Please wait while we load your content...
Please wait while we load your content...