Peralkylated imidazolium carbonate ionic liquids: synthesis using dimethyl carbonate, reactivity and structure†
Abstract
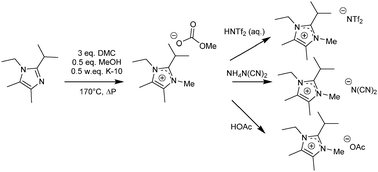
Tri- and tetra-alkylimidazoles are quaternised into their corresponding ionic liquids with dimethyl carbonate. Upon metathesis of the obtained methyl carbonate salts, only gaseous by-products are generated. These methyl carbonate salts can be transformed into hydrogen carbonate salts by reaction with water. The salts containing a carbonate anion are very alkaline, which results in a hydrogen/deuterium exchange on the anion and some of the cation protons, depending on the substitution. Moreover, the crystalline 1-ethyl-3,4,5-trimethylimidazolium hydrogen carbonate formed carboxylate species upon dissolution. In particular, the carboxylate was able to regenerate the carbene and in the presence of chloroform, this led to the formation of the chloride salt.

 Please wait while we load your content...
Please wait while we load your content...