The copper transporter 1 (CTR1) is required to maintain the stability of copper transporter 2 (CTR2)
Abstract
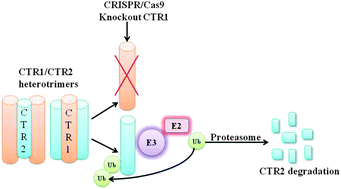
Mammalian cells have two influx Cu transporters that form trimers in membranes. CTR1 is the high affinity transporter that resides largely in the plasma membrane, and CTR2 is the low affinity transporter that is primarily associated with vesicular structures inside the cell. The major differences between CTR1 and CTR2 are that CTR1 contains a HIS/MET-rich domain N-terminal of the METS that participate in the first two stacked rings that form the pore, and a longer C-terminal tail that includes a Cu binding HIS–CYS–HIS (HCH) motif right at the end. It has been reported that CTR1 and CTR2 are physically associated with each other in the cell. We used the CRISPR-Cas9 technology to knock out either CTR1 or CTR2 in fully malignant HEK293T and OVCAR8 human ovarian cancer cells to investigate the interaction of CTR1 and CTR2. We report here that the level of CTR2 protein is markedly decreased in CTR1 knockout clones while the CTR2 transcript level remains unchanged. CTR2 was found to be highly ubiquitinated in the CTR1 knock out cells, and inhibition of the proteasome prevented the degradation of CTR2 when CTR1 was not present while inhibition of autophagy had no effect. Re-expression of CTR1 rescued CTR2 from degradation in the CTR1 knockout cells. We conclude that CTR1 is essential to maintain the stability of CTR2 and that in the absence of CTR1 CTR2 is degraded by the proteasome. This reinforces the concept that the functions of CTR1 and CTR2 are inter-dependent within the Cu homeostasis system.

 Please wait while we load your content...
Please wait while we load your content...