Protection from neurodegeneration in the 6-hydroxydopamine (6-OHDA) model of Parkinson's with novel 1-hydroxypyridin-2-one metal chelators†
Abstract
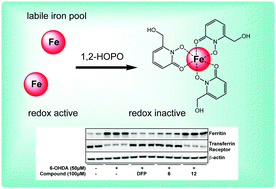
Brain iron accumulation has been associated with inciting the generation of oxidative stress in a host of chronic neurological diseases, including Parkinson's disease. Using the catecholaminergic neurotoxin 6-hydroxydopamine to lesion cellular dopaminergic pathways as a model of Parkinson's disease in culture, a selection of 1-hydroxypyridin-2-one (1,2-HOPO) metal chelators were synthesized and their neuroprotective properties were compared to the 3-hydroxypyridin-4-one; deferiprone (3,4-HOPO; DFP). Protection against 6-OHDA and iron insult by the novel compounds 6 and 9 was comparable to DFP. Iron associated changes by 6-OHDA imply that the neuroprotective capacity of these compounds are due to chelation of the neuronal labile iron pool and the requirement of the iron binding moiety of compound 6 for efficacy supported this hypothesis. In conclusion, two novel 1,2-HOPO's and DFP have comparable neuroprotection against Parkinsonian-associated neurotoxins and supports the continued development of hydroxypyridinone compounds as a non-toxic therapeutic agent in the treatment of neurodegenerative disease.

 Please wait while we load your content...
Please wait while we load your content...