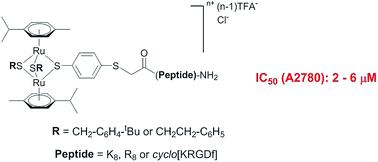
In order to improve the water-solubility of dinuclear thiolato-bridged arene ruthenium complexes, a new series was synthesized by conjugating octaarginine, octalysine, and cyclo[Lys-Arg-Gly-Asp-D-Phe] using chloroacetyl thioether (ClAc) ligation, resulting in cytotoxic conjugates against A2780 human ovarian cancer cells (IC50 = 2–8 μM) and against the cisplatin resistant line A2780cisR (IC50 = 7–15 μM). These metal complexes represent, to the best of our knowledge, the most cytotoxic ruthenium bioconjugates reported so far.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...