Semi-synthesis and anti-tumor evaluation of novel 25-hydroxyprotopanaxadiol derivatives as apoptosis inducing agents†
Abstract
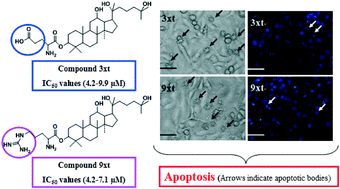
A series of novel 25-hydroxyprotopanaxadiol (25-OH-PPD) derivatives were synthesized by reacting with amino acids, and their in vitro antitumor activities were evaluated on five human tumor cell lines. According to the bioassay results, compounds 3xt and 9xt displayed the most potent anticancer activities with IC50 values ranging from 4.2 to 9.9 μM and 4.2–7.1 μM, respectively. Both of them showed greater growth inhibition than 25-OH-PPD and ginsenoside-Rg3 (an anti-cancer regular drug in China). Further observation of morphological changes, DAPI staining assay and flow cytometry assay demonstrated that compounds 3xt and 9xt could significantly induce apoptosis against human prostatic carcinoma DU145 cells. In conclusion, the current data revealed that compounds 3xt and 9xt may be potential anti-tumor candidates and apoptotic inducers, with low toxicity in normal cells (IOSE144, human ovarian surface epithelial cells). These results may provide useful guidance for developing novel antiproliferative agents.

 Please wait while we load your content...
Please wait while we load your content...