Synthesis of fusidic acid bioisosteres as antiplasmodial agents and molecular docking studies in the binding site of elongation factor-G†
Abstract
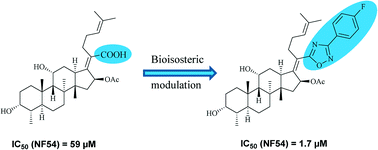
A series of fusidic acid derivatives was synthesized by replacing the carboxylic acid group with various bioisosteres and evaluated in vitro against the chloroquine-sensitive NF54 strain of malaria parasite Plasmodium falciparum. Most of these derivatives showed a 2–35 fold increase in activity as compared to fusidic acid and had a good selectivity index. Further, docking experiments of fusidic acid and the most active derivative 18 within the active site of plasmodial elongation factor-Gs suggested that the binding orientation of 18 is similar to fusidic acid, but with slightly better docking score.

 Please wait while we load your content...
Please wait while we load your content...