PPARα agonists based on stilbene and its bioisosteres: biological evaluation and docking studies†
Abstract
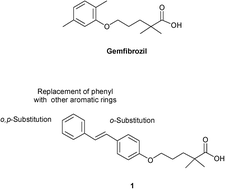
A new series of gemfibrozil analogues conjugated with trans-stilbene were synthesized and evaluated with the aim of developing new PPARα agonists. The phenyls of stilbene were modified by introducing substituents in the ortho or para position and only the distal ring was substituted with naphthyl or heteroaromatic moieties, keeping the dimethylpentanoic skeleton of gemfibrozil unaltered. Two compounds, 5a and 5d, exhibited good activation of PPARα and were also screened for their activity on PPARα-regulated gene CPT1A. Structure-based studies carried out on the active ligands highlighted the dominant role of ligand solvation energy and hydrophobic effect in determining the PPARα activation.

 Please wait while we load your content...
Please wait while we load your content...