Structure-based design, synthesis by click chemistry and in vivo activity of highly selective A3 adenosine receptor agonists†
Abstract
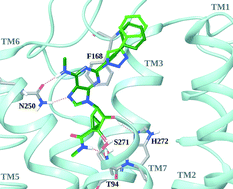
2-Arylethynyl derivatives of (N)-methanocarba adenosine 5′-uronamides are selective A3AR (adenosine receptor) agonists. Here we substitute a 1,2,3-triazol-1-yl linker in place of the rigid, linear ethynyl group to eliminate its potential metabolic liability. Docking of nucleosides containing possible short linker moieties at the adenine C2 position using a hybrid molecular model of the A3AR (based on the A2AAR agonist-bound structure) correctly predicted that a triazole would maintain the A3AR selectivity, due to its ability to fit a narrow cleft in the receptor. The analogues with various N6 and C2-aryltriazolyl substitution were synthesized and characterized in binding (Ki at hA3AR 0.3–12 nM) and in vivo to demonstrate efficacy in controlling chronic neuropathic pain (chronic constriction injury). Among N6-methyl derivatives, a terminal pyrimidin-2-yl group in 9 (MRS7116) increased duration of action (36% pain protection at 3 h) in vivo. N6-Ethyl 5-chlorothien-2-yl analogue 15 (MRS7126) preserved in vivo efficacy (85% protection at 1 h) with short duration. Larger N6 groups, e.g.17 (MRS7138, >90% protection at 1 and 3 h), greatly enhanced in vivo activity. Thus, we have combined structure-based methods and phenotypic screening to identify nucleoside derivatives having translational potential.
- This article is part of the themed collection: Identification and Optimization of GPCR Ligands in the 21st Century

 Please wait while we load your content...
Please wait while we load your content...