Synthesis and cytostatic activity of 7-arylsulfanyl-7-deazapurine bases and ribonucleosides†
Abstract
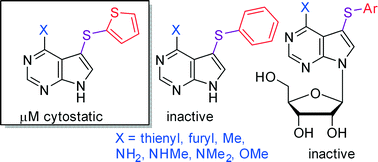
A series of 7-phenylsulfanyl- or 7-(2-thienyl)sulfanyl-7-deazapurine bases bearing diverse substituents at position 6 was prepared through C–H sulfenylation of

 Please wait while we load your content...
Please wait while we load your content...