Oligomerization enhancement and two domain swapping mode detection for thermostable cytochrome c552via the elongation of the major hinge loop†
Abstract
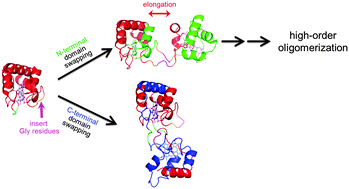
High-order oligomers of Hydrogenobacter thermophilus cytochrome c552 increased with the insertion of more Gly residues between Ala18 and Lys19 at the major hinge loop of the wild-type protein. N-Terminal domain swapping and C-terminal domain swapping were elucidated by using X-ray crystallography for the mutant with the insertion of three Gly residues at the hinge loop.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...