Structural insights into tumor-specific chaperoning activity of gamma synuclein in protecting estrogen receptor alpha 36 and its role in tamoxifen resistance in breast cancer†
Abstract
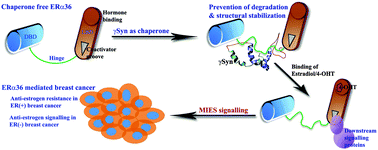
Gamma synuclein (γSyn), a tumor-specific molecular chaperone, protects Hsp90 client proteins like ERα36 and stimulates rapid membrane-initiated estrogen signalling in breast cancer cells. However, the structural perspectives of this tumor-specific chaperone function of γSyn remains unclear. Hence, in this present work, we studied the conformational dynamics of ERα36 in the absence and presence of Hsp90 and γSyn. Results indicate that in a chaperone-free state, ERα36 undergoes an inter-domain movement and exposes the hydrophobic patch of residues that are responsible for binding with ubiquitin. However, independent of Hsp90, γSyn, by establishing transient interactions, prevents interdomain movement, unveils the co-activator binding groove, masks the ubiquitin-binding residues and maintains ‘open’ pocket conformation of LBD. By doing so, γSyn effectively protects ERα36 from degradation and maintains its functional state like Hsp90 based chaperoning machinery but independent of ATP. Our studies also show that the γSyn protected conformation of ERα36 can effectively bind with both estradiol (E2) and 4-hydroxy tamoxifen (4-OHT). Although they exhibit unique binding modes, they maintained the functionally active conformation of ERα36. Interestingly, the molecular dynamics simulation studies showed that 4-OHT, like γSyn, prevented the interdomain movements, primes the co-activator binding groove of ERα36 for complexation with downstream signalling proteins and this mechanism explains its agonist activity and associated anti-estrogen resistance observed in the presence of ERα36. The observed differences in the chaperoning mechanism of γSyn sheds light on its selectivity over Hsp90 in cancer cells, for promoting rapid protection of crucial oncogenic proteins. Based on our findings, we speculate that the compounds, which can hamper association of γSyn with ERα36 and/or can arrest ERα36 in an ubiquitin binding state, would be promising alternatives for treating ERα36 expressed breast carcinomas.

 Please wait while we load your content...
Please wait while we load your content...