Tarantula myosin free head regulatory light chain phosphorylation stiffens N-terminal extension, releasing it and blocking its docking back†
Abstract
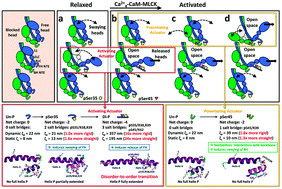
Molecular dynamics simulations of smooth and striated muscle myosin regulatory light chain (RLC) N-terminal extension (NTE) showed that diphosphorylation induces a disorder-to-order transition. Our goal here was to further explore the effects of mono- and diphosphorylation on the straightening and rigidification of the tarantula myosin RLC NTE. For that we used MD simulations followed by persistence length analysis to explore the consequences of secondary and tertiary structure changes occurring on RLC NTE following phosphorylation. Static and dynamic persistence length analysis of tarantula RLC NTE peptides suggest that diphosphorylation produces an important 24-fold straightening and a 16-fold rigidification of the RLC NTE, while monophosphorylation has a less profound effect. This new information on myosin structural mechanics, not fully revealed by previous EM and MD studies, add support to a cooperative phosphorylation-dependent activation mechanism as proposed for the tarantula thick filament. Our results suggest that the RLC NTE straightening and rigidification after Ser45 phosphorylation leads to a release of the constitutively Ser35 monophosphorylated free head swaying away from the thick filament shaft. This is so because the stiffened diphosphorylated RLC NTE would hinder the docking back of the free head after swaying away, becoming released and mobile and unable to recover its original interacting position on activation.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems


 Please wait while we load your content...
Please wait while we load your content...