Compartmentalized calcium signaling triggers subpopulation formation upon platelet activation through PAR1†
Abstract
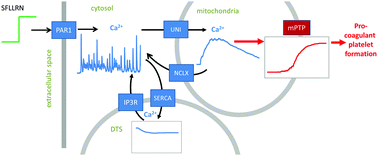
Blood platelets need to undergo activation to carry out their function of stopping bleeding. Different activation degrees lead to a stepped hierarchy of responses: ability to aggregate, granule release, and, in a fraction of platelets, phosphatidylserine (PS) exposure. This suggests the existence of decision-making mechanisms in the platelet intracellular signaling network. To identify and investigate them, we developed a computational model of PAR1-stimulated platelet signal transduction that included a minimal set of major players in the calcium signaling network. The model comprised three intracellular compartments: cytosol, dense tubular system (DTS) and mitochondria and extracellular space. Computer simulations showed that the stable resting state of platelets is maintained via a balance between calcium pumps and leaks through the DTS and plasma membranes. Stimulation of PAR1 induced oscillations in the cytosolic calcium concentrations, in good agreement with experimental observations. Further increase in the agonist level activated the mitochondrial uniporter leading to calcium uptake by mitochondria, which caused the collapse of mitochondrial membrane potential in a fraction of platelets leading to the PS exposure. The formation of this subpopulation was shown to be a stochastic process determined by the small number of activated PAR1 receptors and by heterogeneity in the number of ion pumps. These results demonstrate how a gradual increase of the activation degree can be converted into a stepped response hierarchy ultimately leading to formation of two distinct subpopulations from an initially homogeneous population.

 Please wait while we load your content...
Please wait while we load your content...