Efficiency optimisation of proteins on a chip
Abstract
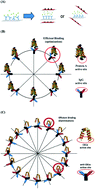
This study elucidates that the protein reorientation on a chip can be changed by an external electric field (EEF) and optimised for achieving strong effective binding between proteins. Protein A and its binding protein immunoglobulin G (IgG) were used as an example, in addition to an anticancer peptide (CB1a) and its antibody (anti-CB1a). The binding forces (BFs) were measured by atomic force microscopy (AFM) with EEFs applied at different angles (EEF°). The optimal angle (OA) of the EEF (OAEEF°) corresponding to the maximum binding force (BFmax) was obtained. The results showed that the BFmax values between IgG/Protein A and anti-CB1a/CB1a were 6424.2 ± 195.3 pN (OAEEF° = 45°) and 729.1 ± 33.2 pN (OAEEF° = 22.5°), respectively. Without an EEF, the BF values were only 730.0 ± 113.9 pN and 337.3 ± 35.0 pN, respectively. Based on these observations, we concluded that the efficient optimisation of protein–protein interaction on a chip is essential. This finding is applicable to the industrial fabrication of all protein chips.

 Please wait while we load your content...
Please wait while we load your content...