Abstract
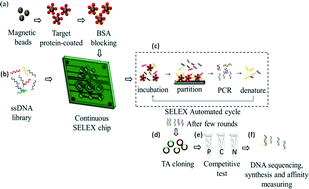
Blood glycated hemoglobin (HbA1c) levels reflecting average glucose concentrations over the past three months are fundamental for the diagnosis, monitoring, and risk assessment of diabetes. It has been hypothesized that aptamers, which are single-stranded DNAs or RNAs that demonstrate high affinity to a large variety of molecules ranging from small drugs, metabolites, or proteins, could be used for the measurement of HbA1c. Aptamers are selected through an in vitro process called systematic evolution of ligands by exponential enrichment (SELEX), and they can be chemically synthesized with high reproducibility at relatively low costs. This study therefore aimed to select HbA1c- and hemoglobin (Hb)-specific single-stranded DNA aptamers using an on-chip SELEX protocol. A microfluidic SELEX chip was developed to continuously and automatically carry out multiple rounds of SELEX to screen specific aptamers for HbA1c and Hb. HbA1c and Hb were first coated onto magnetic beads. Following several rounds of selection and enrichment with a randomized 40-mer DNA library, specific oligonucleotides were selected. The binding specificity and affinity were assessed by competitive and binding assays. Using the developed microfluidic system, the incubation and partitioning times were greatly decreased, and the entire process was shortened dramatically. Both HbA1c- and Hb-specific aptamers selected by the microfluidic system showed high specificity and affinity (dissociation constant, Kd = 7.6 ± 3.0 nM and 7.3 ± 2.2 nM for HbA1c and Hb, respectively). With further refinements in the assay, these aptamers may replace the conventional antibodies for in vitro diagnostics applications in the near future.
 Please wait while we load your content...
Please wait while we load your content...