Thermodynamic and experimental study of the degradation of the red pigment mercury sulfide
Abstract
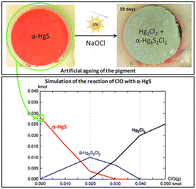
The red pigment mercury sulfide, called cinnabar or vermilion, is well known to suffer from an alteration giving rise to a grey, grey-white or black color at the surface of degraded works of art. This phenomenon can dramatically affect the esthetical value of artworks. This work aims at assessing the factors (light, halides) influencing the instability of red mercury sulfide and understanding (by combining thermodynamic and experimental approaches) the chemical equilibria governing the formation and evolution of the different degradation compounds. From the thermodynamic study of the Hg–S–Cl–H2O system, it was concluded that Hg(0), Hg3S2Cl2, and Hg2Cl2 can be formed from the reaction of α-HgS with ClO(g). In the second part, the artificial ageing experiments presented were carried out on model samples following the conditions assessed in the first part, in order to reproduce natural ageing observed on red mercury sulfide. Similarly to degradation compounds detected on original works of art, mercury chlorine compounds such as calomel (Hg2Cl2) and corderoite (α-Hg3S2Cl2) were identified on the surface of α-HgS model samples, when exposed to light and a sodium hypochlorite solution. Sulfates were detected as well, and more particularly gypsum (CaSO4·2H2O) when Ca was originally present in the model sample. The relationship between color and composition is discussed as well.
- This article is part of the themed collection: Synchrotron radiation and neutrons in art and archaeology

 Please wait while we load your content...
Please wait while we load your content...