A highly stable but highly reactive zinc catalyst for transesterification supported by a bis(imidazole) ligand†
Abstract
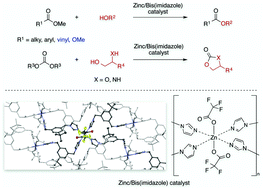
We designed highly active and practical bis(imidazole)/zinc complexes for transesterification reactions. X-ray crystallographic analysis was used to confirm the structures of the zinc complexes and an equivalent of bis(imidazole) ligand was crucial for high catalytic activity. The octahedral zinc complex 8c was prepared in up to a multigram scale by mixing Zn(OCOCF3)2·xH2O and meta-bis(imidazolylmethyl)benzene ligand 7j (two equivalents per zinc ion) and storable under air at room temperature for at least 9 months. The stable nature of the catalyst was amenable to recovery/reuse at least five times without a significant loss of reactivity. The transesterification reaction proceeded without strict reaction conditions, and expanded substrate generalities, including sterically demanding secondary and tertiary alcohols, were applicable. Remarkably, the present zinc catalyst proved highly effective for valuable monomer synthesis from readily available methyl acrylate derivatives. Chemoselective transesterification reactions of unprotected-amino alcohols were also achieved, using not only a simple methyl ester but also an unprecedented dimethyl carbonate.

 Please wait while we load your content...
Please wait while we load your content...