Plasma membrane permeabilisation by ionic liquids: a matter of charge†
Abstract
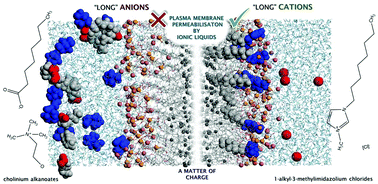
Understanding the mechanisms of toxicity of ionic liquids at the molecular level is crucial for their conscious design as to promote higher acceptability as green solvents in a wide range of applications. In this systematic study, we investigated the effects of three families of ionic liquids on the plasma membrane of the filamentous fungus Aspergillus nidulans. Using fluorescence microscopy and gene expression analysis, we were able to demonstrate that the widely studied 1-alkyl-3-methylimidazolium chlorides with long alkyl substituents cause membrane permeabilisation. In opposition, the biocompatibility of cholinium alkanoates was reinforced here, even though their toxicity also increases with the elongation of the anion. Further investigating the effects of charge on membrane permeabilisation, we observed that a series of alkyl-(2-hydroxyethyl)-dimethylammonium bromides led to permeabilisation of the fungal plasma membrane by increasing the length of one alkyl substituent in the cholinium cation. We hypothesise that the chemical nature of the plasma membrane, which presents a heterogeneous charge distribution along its surface, is pivotal for the membrane permeabilising effects of ionic liquids. This study may inspire a new trend in the ionic liquid field: to give preference for the development of compounds carrying functionalised anions as greener alternatives to those carrying functionalized cations.

 Please wait while we load your content...
Please wait while we load your content...