Free-radical conversion of a lignin model compound catalyzed by Pd/C†
Abstract
Efficient cleavage of a C–O bond in lignin and its model compounds is of great importance for transformation of lignin into fuel and value-added chemicals. In this work, we explored the transformation of a lignin model compound benzyl phenyl ether (BPE) at 150 °C by using Pd/C as the catalyst under an argon atmosphere in the presence of Na2CO3 and N-methyl-2-pyrrolidone (NMP). The effects of different reaction parameters such as the amounts of Pd/C, Na2CO3 and NMP as well as reaction times were investigated. It was found that Pd/C played a key role in the conversion of BPE. At the same time, Na2CO3 and NMP also promote the transformation effectively. The yields of phenol and toluene reached 71.6% and 50.4%, respectively. Analytical results of electron paramagnetic resonance (EPR) indicated that the reaction proceeded through a free-radical reaction mechanism. Control experiments indicated that direct pyrolysis of BPE was the main route to generate the target products.
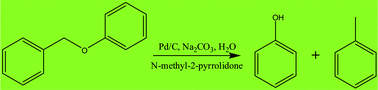

 Please wait while we load your content...
Please wait while we load your content...