Environmental safety of cholinium-based ionic liquids: assessing structure–ecotoxicity relationships†
Abstract
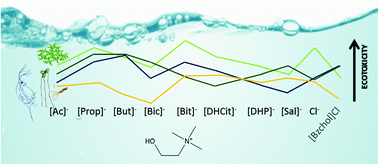
Ionic liquids (ILs) are innovative solvents that can be tuned for their specific application through the selection, or functionalization, of the cation and the anion. Although the cation has been assumed as the main driver of toxicity, the importance of the anion must not be underestimated. This study considers a series of cholinium based ILs aiming at assessing the effects of the functionalization of the cation and the anion on their ecotoxicity. These effects were assessed using three biological models, the microalgae Raphidocelis subcapitata, the macrophyte Lemna minor and the cladoceran Daphnia magna, representing aquatic ecosystems, a major putative recipient of ILs due to their high water solubility. Since the toxicity trends fluctuated depending on the biological model, the results were integrated with previous data through a species sensitivity distribution approach in an attempt to provide a useful safety variable for the design of eco-friendlier ILs. The results reported here challenge some heuristic rules previously proposed for the design of ILs, in particular in what concerns the side-chain effect for the cholinium ILs, and the notion that cholinium-based ILs are inherently safe and less environmentally hazardous than most conventional solvents. Moreover, it was confirmed that structural changes in the ILs promote differences in toxicity highlighting the importance of the role of the anion in their toxicity. Different biological systems yielded different toxicity trends across the IL series tested, also distinct from previous data retrieved with the bacteria V. fischeri; such a novel integration effort challenges the suitability of establishing structure–ecotoxicity relationships for cholinium-based IL design. Overall, this study reinforces the need to perform complete ecotoxicological characterisation before assuming ILs as suitable, environmentally compatible, alternative solvents.

 Please wait while we load your content...
Please wait while we load your content...