Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles†
Abstract
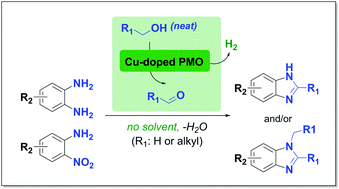
Benzimidazoles and N-methylbenzimidazoles were synthesized by simply heating 1,2-diaminobenzenes in supercritical methanol over copper-doped porous metal oxides. These catalysts were derived from synthetic hydrotalcites that only contain earth-abundant starting materials. The carbon equivalents needed for the construction of the benzimidazole core originated from the solvent itself, which is known to undergo reforming to hydrogen and carbon monoxide through the formation of a formaldehyde intermediate. A variety of 1,2-diaminobenzenes were converted to the corresponding mixtures of benzimidazoles and N-methylated analogues in good yields. Interestingly, the more challenging, but readily available 2-nitroanilines, which require an additional reduction step prior to cyclization, could also be successfully converted to benzimidazoles in high selectivity. Furthermore, various other alcohols were applied besides methanol, to obtain 2-alkyl- and 1,2-dialkylbenzimidazoles. Preliminary mechanistic insights into the origins of N-alkylation as well as the reactivity of the nitro derivatives are discussed.
- This article is part of the themed collection: Green solvents for synthesis

 Please wait while we load your content...
Please wait while we load your content...