Suzuki cross-coupling in aqueous media†
Abstract
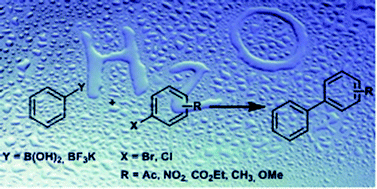
We report a simple and efficient procedure for the ligand-free as well as ligand-assisted Suzuki reaction in both pure water and aqueous media. The cross-coupling reactions proceed successfully using phenylboronic acid or potassium phenyltrifluoroborate as a nucleophilic coupling partner. The method can be effectively applied to both activated and deactivated aryl halides yielding quantitative conversions. The catalytic activity of couplings performed in pure water increases when utilizing supramolecular additives, but decreases under standard phase-transfer conditions. Finally, the palladium loading is reducible from 3.0 mol% to 0.4 mol% without any loss of conversion.

 Please wait while we load your content...
Please wait while we load your content...