Friedel–Crafts alkylation catalysed by GaCl3-based liquid coordination complexes
Abstract
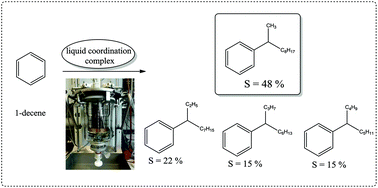
Friedel–Crafts alkylation of benzene with 1-decene was catalysed by a new family of liquid Lewis acids: liquid coordination complexes (LCCs). LCCs are prepared by mixing a metal halide (e.g. GaCl3) and a donor molecule (e.g. N,N-dimethylacetamide, urea, or trioctylphosphine oxide), with the metal halide typically used in excess. This leads to the formation of a eutectic mixture comprised of charged and neutral species in a dynamic equilibrium. GaCl3-based LCCs were used in catalytic amounts, giving high reaction rates under ambient conditions, with selectivities to 2-phenyldecane superior to those previously reported in the literature. The influence of reaction conditions and catalyst composition on the reaction rate and selectivity was investigated. Optimised reaction conditions were suggested. This exploratory study offers promise with regard to the development of safer, LCC-based alternatives to HF in industrial alkylations.

 Please wait while we load your content...
Please wait while we load your content...