Biodegradable betaine-based aprotic task-specific ionic liquids and their application in efficient SO2 absorption†
Abstract
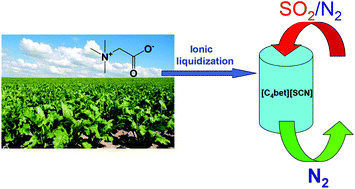
Utilization of cheap and natural resources is an important topic in green chemistry. A new class of betaine-based aprotic task-specific ionic liquids (ILs), including [C4bet][TFSI], [C4bet][DCA], [C4bet][SCN], [1O2bet][TFSI], [1O2bet][DCA], [2O2bet][TFSI], and [2O2bet][DCA], has been designed and prepared through a new ionic liquidization strategy from the renewable natural resource betaine. Their physicochemical characteristics, including spectral properties, density, viscosity, melting point, glass transition temperature, thermal stability, conductivity and electrochemical window, have been comprehensively studied. They were first applied as new absorbents for SO2 capture, and [C4bet][SCN] exhibited the maximum absorption capacity of 0.93 molSO2/molILs at 20 °C and 1 atm (SO2/N2 = 10% vol.) with rapid absorption rates in 21 min. Moreover, the effect of temperature, pressure, and water content on the absorption performance of SO2 was investigated. The possible absorption mechanism was studied using FT-IR, NMR and quantum chemical calculation. In addition, the [C4bet][SCN] can maintain the high absorption capacity and the rapid absorption rates for 25 absorption/desorption cycles.

 Please wait while we load your content...
Please wait while we load your content...